Newly discovered ice properties show how our universe might produce organic molecules in deep space
Laboratory astrophysicists led by Jiao He (Max Planck Institut for Astronomy) have found a new mechanism that could explain how complex organic molecules form in interstellar clouds – a key open question in astrochemistry. The mechanism depends on unusual properties uncovered in experiments with artificial "cosmic chemistry labs" – simplified models of the ice-covered dust grains that facilitate chemical reactions in outer space. The transition to so-called "polycrystalline" carbon monoxide ice could allow embedded molecules and radicals to cluster within the ice, paving the way for more complex chemical reactions. The results have been published in the Astrophysical Journal Letters.
Laboratory experiments simulating how ice layers form on minute cosmic grains of dust have provided a possible answer for one of the key open questions of deep-space chemistry: how complex organic molecules can form in the rarefied environment of interstellar molecular clouds. Such molecules, transported to Earth by meteorites, might be one of the ingredients that led to the formation of life on Earth, and possibly on other suitable planets.
Organic molecules between the stars
The gigantic voids of space between stars are almost empty, but not quite – and in the thin clouds of gas and dust one can find there, astronomers have discovered more and more complex molecules over the past decades or so. Curiously, quite a number of the roughly 200 known interstellar molecule species in interstellar space are organic, that is: they contain carbon atoms bound to hydrogen atoms. Organic molecules can form highly complex structures, and here on Earth, molecules of that kind are the basis of life as we know it.
Not surprisingly, that has led to the question whether or not interstellar organic molecules could in any way be connected with the origins of life on Earth, and possibly of life on other planets. One possible scenario involves meteorites transporting organic interstellar molecules into small ponds here on Earth to create the conditions for life to emerge (cf. this MPIA press release).
Introducing tiny cosmic chemistry labs
But how can complex organic molecules form in interstellar space in the first place? The molecular clouds that can be found between the stars have enormously low densities. Even the densest such clouds, with about a hundred thousand gas particles per cubic centimeters, correspond to that engineers would call an extremely good "Ultra-High Vacuum" as they try to emulate such conditions here on Earth. Ordinary chemical reactions, with molecules or atoms bumping into each other to form compound, are much too rare under such conditions to produce anything but very simple molecules.
In the 1960s, astronomers interested in interstellar chemistry began to develop the idea that interstellar dust grains could serve as "cosmic laboratories," which would facilitate more complex chemical reactions. Such dust grains, based either on carbon or silicates and less than one millionth of a meter in diameter, typically form in the outer layers of cool stars or in the aftermath of supernova explosions. In an interstellar molecular cloud, such dust grains would accumulate an outer layer of water ice, and such icy layers could then serve as a tiny cosmic chemistry labs.
The difficulties of forming more complex molecules
The ice cover of cosmic dust particles typically has an onion-like structure, with tens of consecutive layers. The inner layers consist mostly of water ice, but also contain molecules such as carbon dioxide (CO2), ammonia (NH3), methane (CH4) and others. Chemistry-wise, the outer layers are much more interesting. Here, the main ingredient is carbon monoxide ice (CO), mixed with other components such as the organic compounds methanol (CH3OH) or formaldehyde (H2CO). It may also contain hydrogen and oxygen atoms, as well as compounds called "radicals" that are particularly likely to take part in chemical reactions: hydroxyl (OH), formyl (HCO), methoxy (CH3O), hydroxymethyl (CH2OH) and others.
Earlier laboratory experiments showed conclusively that chemical reactions between these reactive species in the CO-rich ice layers lead to the formation of numerous interesting complex organic molecules, including methyl formate (CH3OCHO), glycolaldehyde (HCOCH2OH), and ethylene glycol [(CH2OH)2] – even at temperatures as low as 10 Kelvin, as is typical in interstellar molecular clouds. But a big open question was the "how" of it all. In order to form more complex molecules, the chemical reactants embedded in the ice would need to move to the same place. Chemical reactions, after all, cannot take place at a distance. That is where previous models ran into difficulties – and where the new work by He and his colleagues provides a possible solution.
Conditions for molecular mobility
Molecules embedded in solid ice are not completely frozen in place. In any piece matter with a non-zero temperature, atoms are constantly wiggling around a bit, and ever so often, this wiggling allows embedded molecules to a new position – either by "squeezing through" or because one of the myriad bonds holding the ice together is temporarily loosened or even broken. This occasional motion-through-wriggling is called “diffusion”.
One of the most important topics in laboratory astrochemistry is to quantify the diffusion rate of various atoms, molecules, and radicals on and within the ice mantle of a dust grain. Results for solid ice have been rather discouraging. With the exception of small hydrogen atoms and molecules, diffusion in ice is exceedingly slow at 10 Kelvin, the typical temperature of interstellar molecular clouds. This is a major problem for the formation of more complex molecules. Unless the reactants just happen to sit next to each other by rare chance, the necessary chemical reactions simply won't happen under those conditions.
Simulating ice on dust grains
That was the situation when Francis Toriello, then a PhD student at Syracuse University and his supervisors Jiao He (initially at Leiden University, later at MPIA) and Gianfranco Vidali (Astrophysics and Surface Science Laboratory at Syracuse University) set out to study the formation of CO ice layers on dust grains in more detail.
Following an experimental design developed by He, Toriello created an ultrahigh vacuum environment that contained a small gold-coated copper disk, 13 mm in diameter. The disk, which represents the surface of a cosmic dust grain, is attached to an external cooling apparatus and can be cooled down to temperatures as low as 5 K in a controlled manner. By piping water vapor or CO gas into the chamber, the researchers can systematically grow layers of water ice, or CO ice, on the disk.
The growing ice layers are then observed with an infrared spectrometer: a lamp shines infrared light onto the ice, and the reflected light is analyzed. The way the material absorbs light at specific wavelengths provides some information about the properties of the ice.
A crucial phase transition
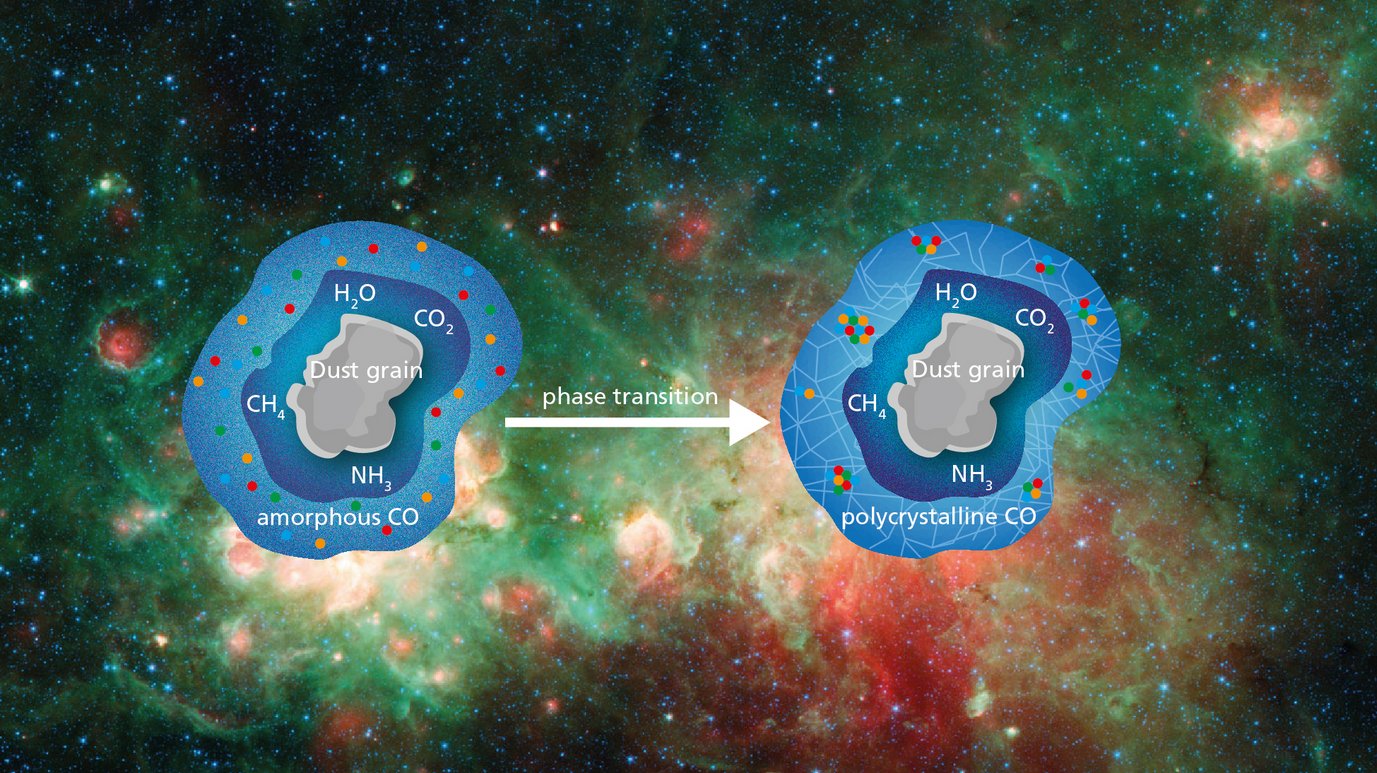
In a series of experiments, the researchers began by preparing a multilayered water ice "core," and then, with the icy core at a temperature of 6 Kelvin, proceeded to deposit carbon monoxide ice layers of differing thickness on top. Afterwards, they warmed the sample to 20 Kelvin, carefully monitoring the infrared spectra the whole time.
At around 10 Kelvin, a typical temperature within dense interstellar clouds, something interesting happened. The infrared spectrum shifted, in a way that the researchers interpret as a phase transition: Below that temperature, the carbon monoxide ice was in an amorphous phase, with the CO molecules sticking together every which way. Above that temperature, the phase changes, most likely into a so-called polycrystalline phase: an accumulation of numerous small CO ice crystals.
A phase transition that helps with mobility
In order to find out what this meant for the role of the CO ice as a "cosmic lab," the researchers set up a second version of the experiment where a bit of carbon dioxide (CO2) was mixed in as the initial CO ice layers were created. The CO2 was meant to represent, more generally, any kind of reactant one might find sticking to the ice layer of cosmic dust. Below 10 Kelvin, all was as expected: The CO2 molecules were stuck in the ice separately, and unable to come together (and, in consequence, to take part in chemical reactions).
But during the time that the phase transition occurred, the situation changed drastically. Afterwards, the infrared spectrograph indicated a strong signal from clusters of CO2 molecules, which had evidently found each other and clumped together. During the transition phase to the polycrystalline form of CO ice, CO2 molecules, and presumably other radicals and molecules, are apparently able to move around in the ice, creating suitable conditions for chemical reactions to take place!
Linking more general considerations about phase transitions with their experimental results, the researchers then went on to consider what the phase transition would mean for ice-covered dust grains in gigantic interstellar clouds. They concluded that the transition to the polycrystalline state should be the norm, not the exception, in such clouds: In the very first stages of star formation, when parts of the cloud begin to collapse, and in consequence to heat up, CO ice on dust grains in the vicinity would become polycrystalline, and during the radicals and molecules would be able to wander more freely, and clump together.
How cosmic chemistry labs could work
Extrapolating from CO2 to more complex reactants, that could explain the efficiency of dust-grain-based cosmic chemistry labs: Over time, cosmic dust grains would collect ice and stray radicals or molecules. Once star formation sets in, the phase transition would allow those radicals and molecules to clump together, providing the conditions in which chemical reactions could take place, and where more complex molecules could form – molecules that might, eventually and after being transported to a newly-formed planet by way of meteorites, play a role in the origin of new life.
In light of the experiments completed so far, this is an intriguing and appealing scenario. Potentially, this way of "running" cosmic chemistry labs is of considerable importance for the emergence of complex organic molecules, and eventually life. But there is still a long way to go before the evidence is strong enough for the scenario to become widely accepted within the scientific community.
Next, Jiao He, who had since moved to Heidelberg as head of the newly founded MPIA Origins of Life laboratory, together with his colleagues, are planning to run a version of the experiment where radicals and molecules other than CO2 are subjected to the phase transition. If this leads to the same clustering effect, that would be another step towards establishing the role of the transition to polycrystalline CO ice in the astrochemistry of interstellar clouds.
Background information
The results described here have been published as J. He et al., "Phase transition of interstellar CO ice," in the Astrophysical Journal Letters.
This paper is part of a collaboration between Syracuse and MPIA which is now continued by operating a new and extended version of the Syracuse experiment at MPIA. The MPIA researchers involved are Jiao He and Thomas Henning, in collaboration with Francis Toriello, Shahnewaz M. Emtiaz and Gianfranco Vidali (all Syracuse University). Vidali is joining MPIA as a guest senior scientist.
