Research Projects
Sugar synthesis from irradiation of interstellar ice analogs
Recent experiments have demonstrated the synthesis of sugars under low-temperature conditions, leading to a productive collaboration with the Meinert group at Nice University and the mass spectrometry group at the University of Jena. We are exploring strategies to enhance sugar production by identifying suitable catalysts, either involving ammonia or the properties of the underlying dust grain surface.
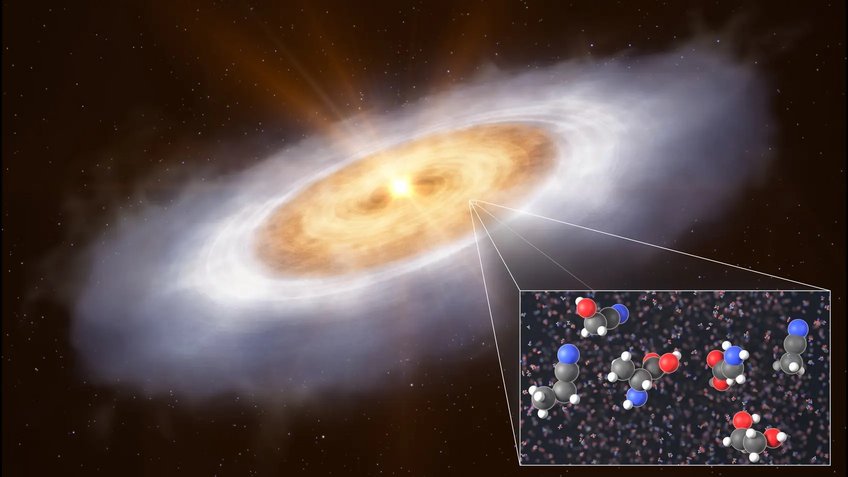
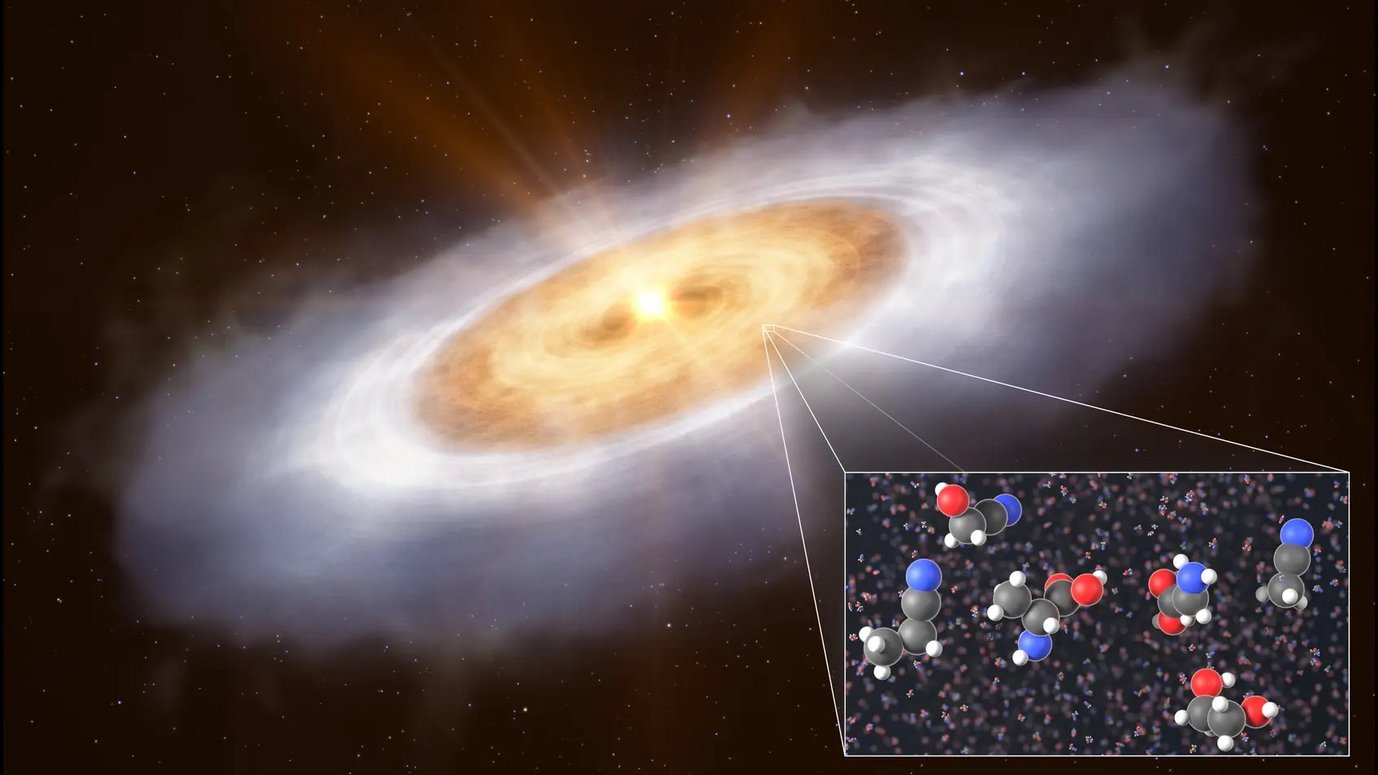
Identification of a range of COMs toward the Protoplanetary Disk of V883 Ori
Together with the observers of the group, we identified precursors to sugars and amino acids in a planet-forming disk, indicating that such disks not only retain but also build upon complex organic molecules from earlier stages of evolution.
References:
A. Fadul, K. R. Schwarz, T. Suhasaria , J. K. Calahan, J. Huang, M. L. R. van't Hoff (2025). A deep Search for Ethylene Glycol and Glycolonitrile in the V883 Ori Protoplanetary Disk. The Astrophysical Journal Letters, 988, L44. Fulltext Link
A. Fadul, K. R. Schwarz, M. L. R. van’T Hoff, J. Huang, J. B. Bergner, T. Suhasaria, J. K. Calahan (2025). A deep search for Complex Organic Molecules toward the protoplanetary disk of V883 Ori. The Astronomical Journal 169 (6), 307. Fulltext Link
Infrared spectra of interstellar ices on realistic dust grain analogs
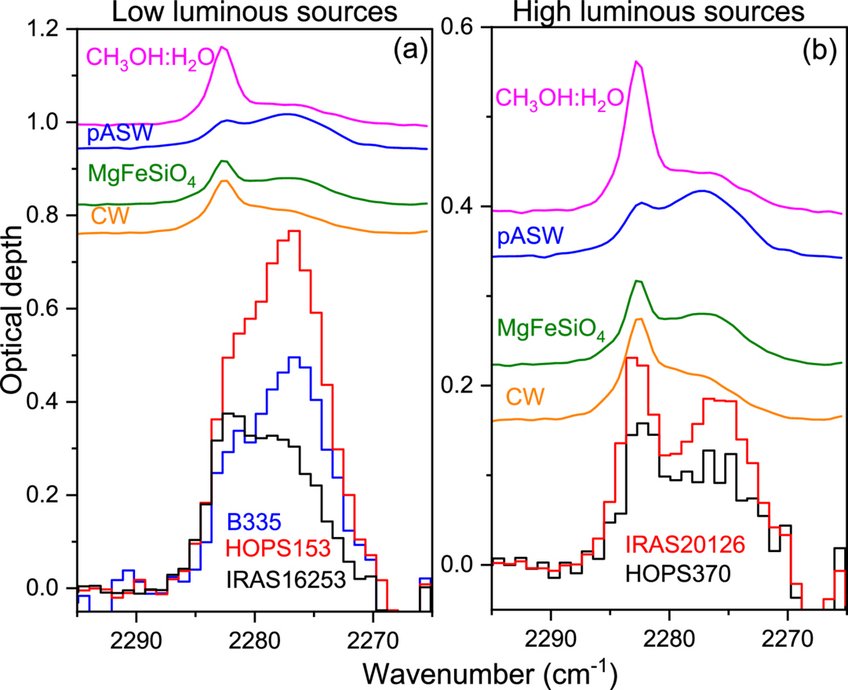
Carbon dioxide ice on interstellar dust grains behaves differently when deposited on amorphous silicate grain analogs than metal. Using RAIRS, the study shows that silicate surfaces relax typical selection rules, producing CO₂ infrared features that better match astronomical observations. When CO₂ is deposited on CO or CH₄ ices over silicate, it crystallizes at lower temperatures. CO₂ also remains bound to silicate surfaces longer during warming. A newly observed split in the ¹³CO₂ infrared feature signals the start of diffusion, and this laboratory profile closely matches JWST observations around young protostars.

T. Suhasaria, V. Leuschner, C. Jaeger, C. Gieser, T. Henning (2025). CO2 infrared spectra on silicate dust grain analogs: Implications for JWST observations. The Astrophysical Journal Letters, 988, L27. Fulltext link
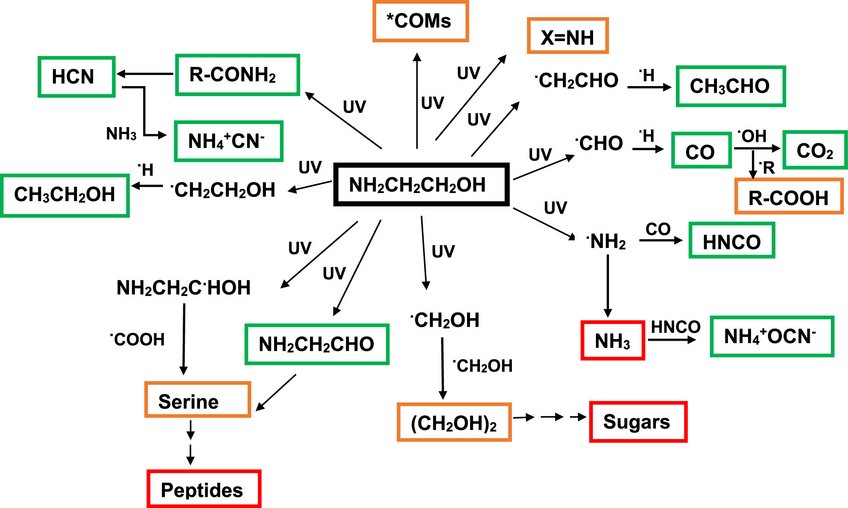
UV Processing of Ethanolamine: Potential Precursors to Sugar and Peptide Derivatives
Ethanolamine (EA), recently detected in molecular clouds, was studied in laboratory ice analogs to assess its stability and chemical role in space. UV irradiation experiments at 10 K show that EA is moderately photostable, with a destruction cross section of ~5 × 10-18 cm² and a half-life of ~65 million years in dense clouds. Spectroscopic analysis reveals multiple photoproducts, and warming the irradiated ice tentatively produces ethylene glycol and serine, indicating that EA may help form complex prebiotic molecules. High-mass fragments also suggest additional complex organics, supporting EA as a potential precursor to biologically relevant chemistry in the interstellar medium.
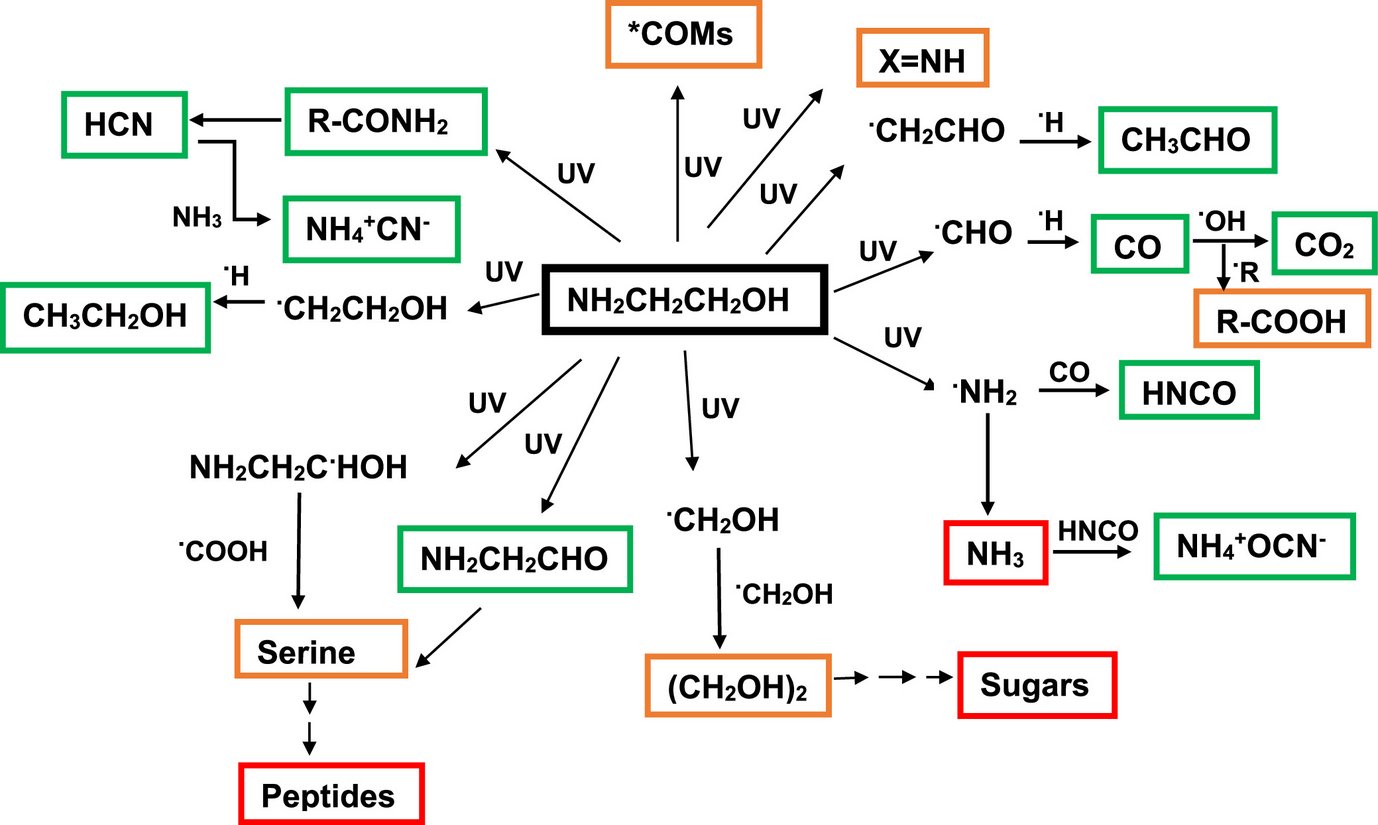
Reference:
T. Suhasaria, S. M. Wee, R. Basalgète, S. Krasnokutski, C. Jäger, K. Schwarz, Th Henning (2025). Ly-processing of solid-state Ethanolamine: Potential Precursors to Sugar and Peptide Derivatives. The Astrophysical Journal, 982 (1), 48. Fulltext Link